
PATHFAST®
Analyzer
The PATH to better cardiac care starts here
When every minute matters
Trust the first point-of-care (POC) high-sensitivity cardiac troponin (hs-cTn) assay in the U.S.
Learn more
Laboratory excellence, propelling ED performance
The PATHFAST Analyzer accelerates urgent cardiac care decisions in the Emergency Department (ED) with reliable, lab-quality testing at the point of care.
PATHFAST meets ED goals of accuracy, efficiency & flexibility
Results available in under 17 minutes
Flexibility to run up to 6 tests at once
Space-saving benchtop design for the ED or STAT lab
User-friendly operation
Eliminating preanalytical delays associated with core-laboratory testing has been shown to:

When every minute matters
Trust the first POC hs-cTn assay in the U.S.
PATHFAST hs-cTnI-II
With PATHFAST, EDs can access an optimal solution to accelerate the assessment and disposition of patients with suspected acute coronary syndrome (ACS).
hs-cTn is the preferred assay for aiding in the diagnosis of acute myocardial infarction (AMI).2
Lab-quality results
- 6% CV at the 99th percentile cutoff of 29 ng/L3
- 4.1 ng/L limit of quantitation (LoQ, I.e. functional sensitivity)3
When and where you need them
- Results in 17 minutes within care pathway
- Run up to 6 tests at once
Optimizing ED performance
- Proven performance in 0/1, 0/2, and 0/3 h accelerated diagnostic protocols4–6
Access to quicker test results can help clinicians identify urgent cardiac concerns sooner, reduce length of stay, and improve patient satisfaction.
Discover more resources
Visit the online resource center for additional education and support materials.
Visit resource center
NT-proBNP is used to aid in the diagnosis of patients with suspected congestive acute heart failure (CHF). Rapid results at the point of care inform rapid risk stratification and admission decisions.
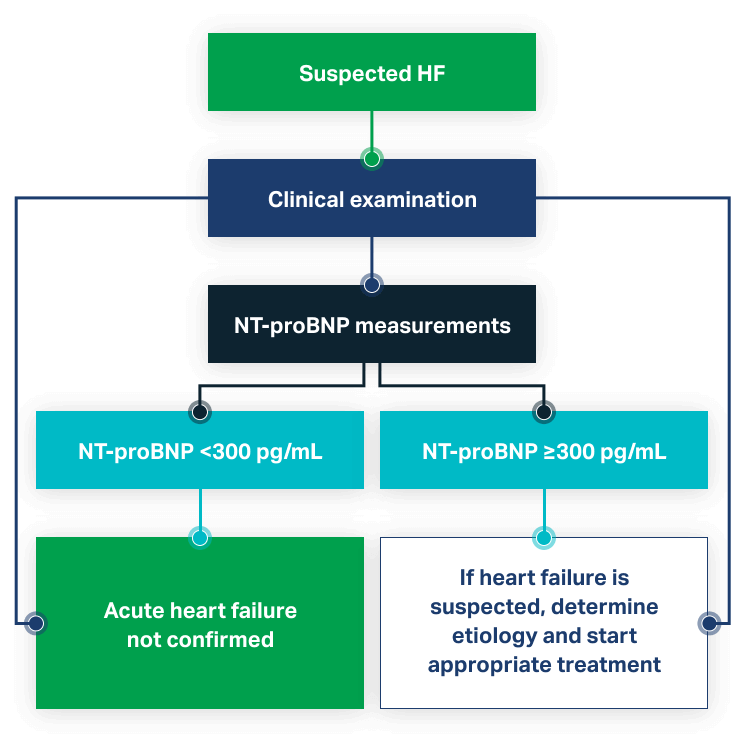
- More accurate diagnosis and improved risk stratification for patients with acute dyspnea7
- Strongest independent predictor of a final diagnosis of acute CHF8
- NT-proBNP plus clinical judgment is superior to either alone8

Discover the best of both worlds
hs-cTn-II + NT-proBNP
PATHFAST is the only point-of-care system to offer both hs-cTnI-II and NT-proBNP, two gold-standard cardiac biomarker tests, on a single platform.
A complete menu of urgent STAT tests
PATHFAST facilitates decisions for a range of assays including:
- hs-cTnI-II
- cTnI-II
- D-Dimer
- CK-MB
- NT-proBNP
- hs-CRP
- Myoglobin
Proactive support every step of the way
Polymedco is committed to being one of the easiest companies to do business within healthcare. We work closely with laboratorians, clinicians, and administrators to maximize the value of our tests
Learn more about the dedicated support you can expect from a PATHFAST partnership
Learn more
Technical specifications
| Instrument type | Benchtop Analyzer |
| Throughput | Up to 6 samples or assays per run |
| Measuring time | <17 minutes for to up to 6 simultaneous samples |
| Sampling material | Whole blood, plasma |
| Measuring principle | Chemiluminescence enzyme immunoassay technology (CLEIA) combined with proprietary Magtration® technology |
| Reaction temperature | 37.5° C |
| Sample volume | 100 µL |
| Wavelength | 300 – 650 nm |
| Data storage | Patient data: 1000 |
| QC data | 1800 |
| CAL data | 300 |
| Data transfer | ASTM standard |
| Dimensions | 13.5 in. W x 22.4 in. D x 18.7 in. H |
| Weight | 62 lbs |
| Electrical requirements | 100 – 240 Vac |
| Monitor/keyboard | LCD Touchscreen |
| Printer/PC | Integrated |
| Interface | RS-232C |
| Calibration | Factory calibration, 2-point calibration every 4 weeks |
| 24-h operation (standby) |  |

Ordering information
Contact us
Learn more about the dedicated support you can expect from a PATHFAST partnership
Learn more

Thank you for your submission. Someone will be in touch soon.
References:
- Goyder C, Tan PS, Verbakel J, et al. Impact of point-of-care panel tests in ambulatory care: a systematic review and meta-analysis. BMJ Open. 2020;10(2):e032132. doi:10.1136/bmjopen-2019-032132
- Kontos, M, de Lemos, J. et al. 2022 ACC Expert consensus decision pathway on the evaluation and disposition of acute chest pain in the emergency department: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(20):1925-1960. doi:10.1016/j.jacc.2022.08.750
- Point of care cardiac troponin I and T assay analytical characteristics. IFCC Committee on Clinical Applications of Cardiac Bio-Markers. Accessed March 13, 2024. https://ifcc.org/ifcc-education-division/emd-committees/committee-on-clinical-applications-of-cardiac-bio-markers-c-cb/biomarkers-reference-tables/
- Sörensen NA, Neumann JT, Ojeda F, et al. Diagnostic evaluation of a high-sensitivity troponin I point-of-care assay. Clin Chem. 2019;65(12):1592-1601. doi:10.1373/clinchem.2019.307405
- Koechlin L, Boeddinghaus J, Lopez-Ayala P, et al. External validation of the 0/1h-algorithm and derivation of a 0/2h-algorithm using a new point-of-care Hs-cTnI assay. Am Heart J. 2024;268:104-113. doi:10.1016/j.ahj.2023.11.014
- Lehmacher J, Sörensen NA, Twerenbold R, et al. Diagnostic and prognostic value of the sex-specific 99th percentile of four high-sensitivity cardiac troponin assays in patients with suspected myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2024;13(1):3-12.doi:10.1093/ehjacc/zuad131
- Bingisser R, Cairns CB, Christ M, et al. Measurement of natriuretic peptides at the point of care in the emergency and ambulatory setting: Current status and future perspectives. Am Heart J. 2013;166(4). doi:10.1016/j.ahj.2013.06.012
- Januzzi JL, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the Emergency Department (Pride) study. Am J Cardiol. 2005;95(8):948-954. doi:10.1016/j.amjcard.2004.12.033

